Innovation Platform
R&D Center
Shanghai R&D Center
- Small-molecule drug development
- Comprehensive in vitro and in vivo evaluation platforms
- Rapid target validation and execution
- Strong domestic medical and clinical development expertise
Baylink (U.S.)
- Focused on ADC research
- Proprietary linker technologies for FIC/BIC ADCs
- Exploration of novel payloads
Technology Platform
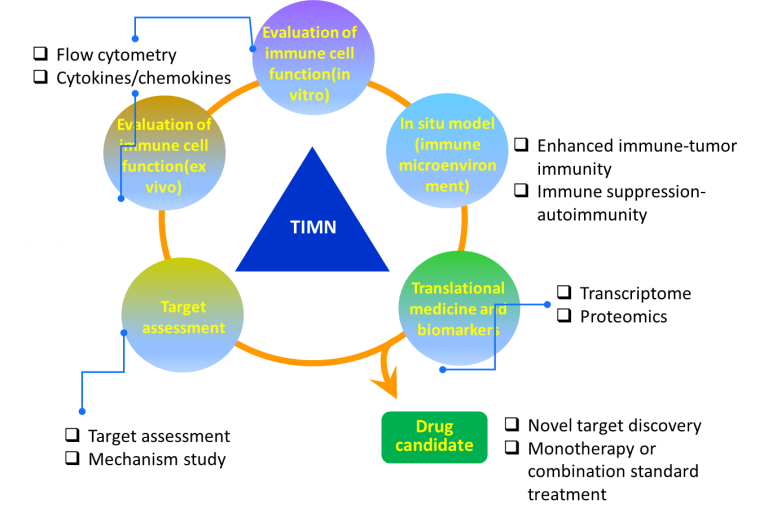
The Targeted Immunomodulation Normalization (TIMN) Platform is designed for developing new drugs for cancer immunotherapy and autoimmune diseases. This platform targets immune-related functional proteins and the immune microenvironment. It integrates target discovery, drug screening , efficacy evaluation, mechanism of action, and translational research to form a comprehensive end-to-end drug discovery system. This TIMN platform can be used to discover drugs that enhance the immune system to normalize the tumor immune microenvironment for anti-tumor treatment and drugs that negatively regulate the immune system to normalize the release of pro-inflammatory factors for treating autoimmune diseases.
The company has advanced/developed APL-1202 (NMIBC, MIBC, etc.), APL-1501 (urological tumors), and APL-1401 (Moderately to severely active ulcerative colitis) with the TIMN platform.
Platform Advantages:
1. Target discovery: Leveraging the tumor immune microenvironment to rapidly identify targets and develop targeted drugs.
2. Drug screening: Utilizing in-situ models to efficiently simulate the patient’s immune microenvironment, enabling the screening of immunomodulators and evaluation of their efficacy and mechanisms of action.
3. Mechanism research: Analyzing the immunomodulatory effects of various subpopulations of cells and cytokines in tissues affected by autoimmune diseases and in-situ tumor tissues.
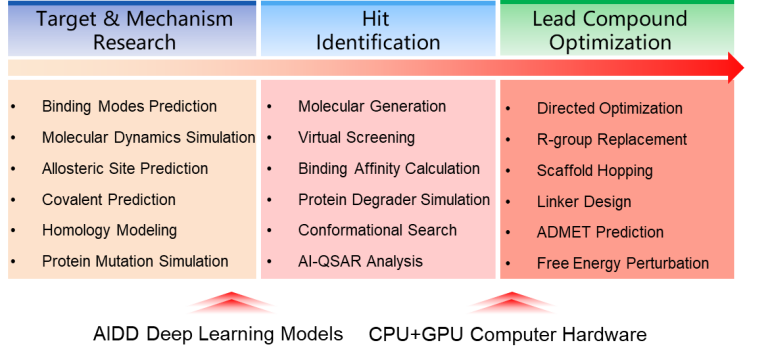
TAIDD is a new-generation Targeted & AI-driven Drug Discovery platform that integrates physical modeling and artificial intelligence technology. TAIDD includes core modules for drug-target binding mode prediction, binding free energy calculation, molecular generation, directed optimization, and prediction of molecular drug-like properties. It provides a comprehensive and multi-angle approach to target-based drug discovery, aiming to help drug researchers obtain preclinical compounds efficiently and cost-effectively. The AI in TAIDD is deeply involved in various stages of preclinical drug development and offers complete and efficient solutions for company’s pipeline. For instance, generative AI based on molecular fragments and medicinal chemistry principles, as well as high-throughput virtual screening based on AI models, are used in hit identification. ADMET prediction using multiple high-quality AI models and water free energy calculations support lead compound optimization. TAIDD emphasizes iterative correction of AI models based on experimental measurements to efficiently assist in compound optimization and design.
The company has advanced/developed APL-2301 (Acinetobacter baumannii infection), APL-2302 (ovarian cancer, breast cancer, etc.), AT-014 (urological tumors), AT-017 (breast cancer), and AT-018 (ovarian cancer, breast cancer, etc.) with the TAIDD platform.
Platform Advantages:
1. AI technology permeates the entire chain of target-based preclinical drug discovery, significantly improving computational efficiency and expanding the chemical space of molecular design with deep learning models.
2. Enable real-time bi-directional interaction between experienced drug researchers and the AIDD platform, achieving seamless human-machine integration.
3. Enhance model reliability through reinforcement learning through experimental measurement data.
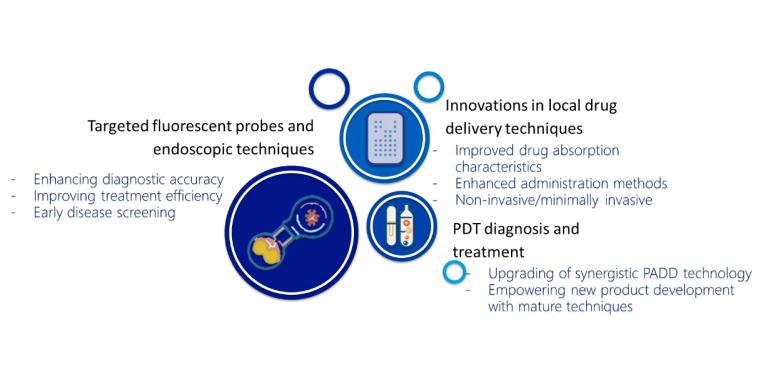
The Drug Device Combination (DDC) platform is a novel medical product development platform built on the Prodrugs Accurate Drug Delivery (PADD) technology platform, leveraging the rich experience in drug development accumulated since its establishment, combined with expertise in drug delivery and medical device development. By utilizing local drug delivery technology, diagnostic or therapeutic drugs can be delivered to the target site, reducing systemic drug exposure, thus improving the safety and efficacy of diagnosis/treatment. In terms of device design, the company fully utilizes its technical reserves and conducts research on functional modules such as targeted fluorescent probes, computer image algorithm combined with endoscopy technology, photodynamic therapy (PDT) integration, and innovative local drug delivery technology. The platform aims to design devices for local drug delivery/drug activation and improved drug dosage forms/administration methods based on human anatomy and physiological characteristics, in order to optimize treatment efficacy while reducing inconvenience and trauma to patients, and enhance compliance. Products are developed based on accumulated research and mature technologies to significantly shorten the development cycle and reduce development costs, thus significantly improving competitiveness in the market, compared to conventional innovative drug development approaches alone.
The company has advanced/developed APL-1702 (Cervical high grade squamous intraepithelial lesions), APL-1706 (NMIBC diagnosis and operation), APLD-2304 (NMIBC diagnosis and operation) with the DDC platform.
Platform Advantages:
1. Improving diagnostic accuracy and treatment efficiency, and expanding the scope of early detection and treatment of diseases.
2. Enhancing treatment efficacy by improving drug absorption and administration methods, achieving diagnosis/treatment in a non-invasive/minimally invasive manner, thereby enhancing patient experience and improving compliance.
3. Leveraging accumulated research and mature technologies to emable the development of new products, synergistically upgrading with PADD technology, and strengthening innovation and application of individualized treatment plans. Compared to the development of class 1 new drugs, DDC products have the advantages of low development costs and short development cycles.